Myanmar profile
Myanmar is a country in Southeast Asia with a population of 52 million. Of those, ∼14 million are younger than age 14 years.1 Hematologic malignancies are now the second most common among pediatric malignancies worldwide after central nervous system tumors, but they are still the most common malignancies in Myanmar. The World Health Organization (WHO) estimated that Myanmar has a total of about 2000 childhood hematologic malignancies per year, according to statistics for our country, but only 594 cases were detected in 2018. Among those, 372 cases were from Yangon Children’s Hospital (YCH).2 That gap between estimation and diagnosis indicates the need to improve outreach diagnosis activities.
Background
YCH is major pediatric hospital serving not only people who live in Yangon but also those whole live anywhere in Myanmar. YCH was established in 1960 as a 60-bed hospital, but thanks to gradual upgrades, it is now operating as a 550-bed hospital. It is also a teaching hospital attached to University of Medicine 1, and it has a variety of specialty units, including a hemato-oncology unit. The hemato-oncology unit is led by pediatric hematologist Aye Aye Khaing. It started as a 16-bed unit, but it has now expanded to 75 beds and is the only hemato-oncology unit in the southern portion of Myanmar. It is also the only hemato-oncology unit that uses flow cytometry to diagnose hematologic malignancies.
Diagnosing hematologic malignancies
Until 2016, all hematologic malignancies were diagnosed primarily by morphology on samples by using Romanowsky stain and other special stains. In August 2016, the hospital laboratory started using a BD FACSCanto II Flow Cytometer (2 lasers, 6 colors) with onsite training by 2 professors from MD Anderson Cancer Center (MDACC) in Houston, Texas. One pathologist from the hospital pathology department was trained at MDACC under the Visitor Training Program (VTP) of the American Society of Hematology (ASH) in 2018. To maintain the quality of our diagnoses, we have monthly online case discussion meetings with St. Jude Children’s Research Hospital (Memphis, Tennessee) and World Child Cancer (United Kingdom). Difficult cases are regularly sent to MDACC professors for their expert opinions. Thus, we have continuous collaboration, support, and training from experts abroad.
Outcomes of flow cytometry
Among 902 bone marrow aspirations taken from January 2017 to December 2018, 295 patients (33%) had leukemia, 335 (37%) were examined for posttreatment checkups, and 272 (30%) had idiopathic thrombocytopenic purpura, hypoplastic, or another hematologic disease. After the 902 bone marrow aspirations were examined, 307 samples were analyzed by flow cytometry, which can now subclassify acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and mixed phenotypes. This level of analysis was not possible before we started using flow cytometry. As a result, the patients are getting better, more specific treatments. Detection of minimal residual disease (MRD) by flow cytometry is our next step, but we still have some limitations.
Role of cytogenetics
Unfortunately, there still is no facility that can provide a diagnosis based on cytogenetics at YCH, although there are a few experts in Myanmar who can perform cytogenetic analyses for research purposes only. Cytogenetics can play a significant role in diagnosing hematologic malignancies, according to WHO Classification. Some samples are sent to a supranational laboratory in a neighboring country, but that procedure is time-consuming and not cost-effective. Thus, we still have a gap in our ability to diagnose certain hematologic diseases.
Conclusion
YCH is advancing in its ability to diagnose hematologic malignancies by using flow cytometry and with the support of international experts. However, diagnosing MRD and performing cytogenetic studies are areas that need to be developed. Providing sustainable service is another important need for diagnosing childhood hematologic malignancies.
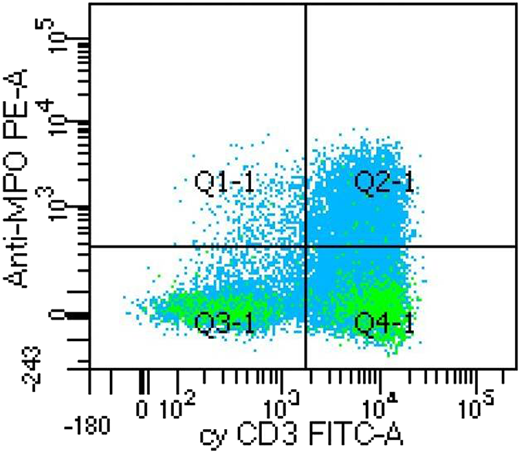
A case of mixed phenotype acute leukemia (T/myeloid) showing dual positive for MPO and cyCD3. Unit of measure is number of dots. Anti-MPO PE-A, myeloperoxidase antibody conjugated with phycoerythrin; cyCD 3 FITC-A, cytoplasmic CD3 antibody conjugated with fluorescein isothiocyanate; Q1-1, MPO positive; Q2-1, both MPO and cyCD3 positive; Q3-1, both MPO and cyCD3 negative; Q4-1, cyCD3 positive.
A case of mixed phenotype acute leukemia (T/myeloid) showing dual positive for MPO and cyCD3. Unit of measure is number of dots. Anti-MPO PE-A, myeloperoxidase antibody conjugated with phycoerythrin; cyCD 3 FITC-A, cytoplasmic CD3 antibody conjugated with fluorescein isothiocyanate; Q1-1, MPO positive; Q2-1, both MPO and cyCD3 positive; Q3-1, both MPO and cyCD3 negative; Q4-1, cyCD3 positive.
Alumni of EndCancer meeting at MD Anderson Cancer Center, Houston, Texas, April 30, 2018.
Alumni of EndCancer meeting at MD Anderson Cancer Center, Houston, Texas, April 30, 2018.
Results of bone marrow morphology analysis
| Disease classification by morphology . | Number of cases . |
|---|---|
| AML | 121 |
| ALL | 158 |
| MPD/MDS | 18 |
| Hemodilute marrow | 35 |
| Active marrow | 118 |
| Metastatic marrow | 21 |
| Hypo/aplastic marrow | 77 |
| ITP | 15 |
| Remission check | 335 |
| Other benign diseases | 4 |
| Disease classification by morphology . | Number of cases . |
|---|---|
| AML | 121 |
| ALL | 158 |
| MPD/MDS | 18 |
| Hemodilute marrow | 35 |
| Active marrow | 118 |
| Metastatic marrow | 21 |
| Hypo/aplastic marrow | 77 |
| ITP | 15 |
| Remission check | 335 |
| Other benign diseases | 4 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ITP, immune thrombocytopenic purpura; MPD/MDS, myeloproliferative/myelodysplastic syndrome.
Results for patient specimens that were subjected to immunophenotyping
| Disease classification by immunophenotyping . | No. of samples . |
|---|---|
| Total No. of samples | 307 |
| AML | 91 |
| ALL | 128 |
| MPAL | 5 |
| No evidence of disease | 62 |
| Inconclusive results | 21 |
| Subclassifications of AML | |
| AML | 58 |
| Myeloid leukemia associated with Down syndrome | 4 |
| APL | 6 |
| FAB AML classification | |
| M4/M5 | 17 |
| M6 | 1 |
| M7 | 5 |
| Subclassifications of ALL | |
| B-cell ALL | 100 |
| T-cell ALL | 28 |
| Disease classification by immunophenotyping . | No. of samples . |
|---|---|
| Total No. of samples | 307 |
| AML | 91 |
| ALL | 128 |
| MPAL | 5 |
| No evidence of disease | 62 |
| Inconclusive results | 21 |
| Subclassifications of AML | |
| AML | 58 |
| Myeloid leukemia associated with Down syndrome | 4 |
| APL | 6 |
| FAB AML classification | |
| M4/M5 | 17 |
| M6 | 1 |
| M7 | 5 |
| Subclassifications of ALL | |
| B-cell ALL | 100 |
| T-cell ALL | 28 |
APL, acute promyelocytic leukemia; FAB, French-American-British [classification system]; M4, acute myelomonocytic leukemia; M5, acute monocytic leukemia; M6, acute erythroid leukemia; M7, acute megakaryoblastic leukemia; MPAL, mixed-phenotype acute leukemia.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ei Phyo Win, Pathology Department, Yangon Children’s Hospital, Dagon Township, Yangon 11191, Myanmar; e-mail: eiphyowin6@gmail.com.